Chitosan Lactate
Water Soluble Chitosan
Product number: D-LP-715583
Chitosan CAS Number: 7012-76-4
Lactic Acid CAS Number: 50-21-5
Chitosan synonym: Deacetylated chitin, Poly(D-glucosamine)
Lactic acid synonym: 2-hydroxypropionic acid, Acidum Lacticum, Milk Acid, Carboxylic
acid
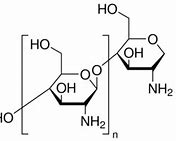
Properties
Deacetylation: >70%
Starting material: Crustacean shell
Appearance: Off white
Mesh size (mm): 60% pass
US $1.33 Per Gram. Price is lower with larger amounts (1Kg+) purchased.
Minimum Order Quantity: 100 grams
For other payment methods, quantities, or more information…
Description
Chitosan Lactate is a high quality grade chitosan salt containing the lactate counter-ion. Unlike its chitosan counterpart, its water soluble. It does this by combining lactic acid onto the amino groups in chitosan without a catalyst. The XRD and 13CNMR results by lactic acid and placement at C2 site in CH. This leads to disruption of the packing in the original CH chains and formation of the amorphous CH salts.
This chitosan’s source material is from the Crustacean shells Sources, specifically from Snow Crab (Chionoecetes opilio).
Chitosan forms gels with multivalent anions and clear solutions that dry to strong, clear films. Its ideal for scaffolds, cell therapy, drug delivery, and biomedical applications.
Its a natural biopolmer that’s biocompatible, biodegradable, antibacterial, an adhesive to tissue, and can be used in a variety of applications, e.g.,
- Biomedical devices
- Drug delivery facilitator
- Wound-healing
- Micro and Nanoparticle encapsulation for controlled release in drug delivery and other therapeutics
- Scaffolding tissue engineering and regeneration
- Pharmaceutical excipient
- Cosmetics
- Anti-microbial, fertilizer and growth elicitor for plants
- Food preservation
- Dietary supplement
Lactic Acid
Lactic acid (2-hydroxypropionic acid, CH3-CHOH-COOH) is the most widely occurring organic acid in nature. Its an alpha-hydroxy acid (AHA) due to its carboxyl group being adjacent to the hydroxyl group. It is used as a synthetic intermediate in many different organic synthesis industries and in various biomedical and biochemical industries. The conjugate base of lactic acid is called lactate.
The chemical behavior of lactic acid is mostly determined by its two functional groups. Besides the acidic character in aqueous medium, the bifunctionality (a terminal carboxylic acid and a hydroxyl group) allows lactic acid molecules to form ‘‘interesters’’ such as the cyclic dimers, the trimers, or longer lactic acid oligomers.
Lactic acid consists of a mixture of 2-hydroxypropionic acid, its condensation products, such as lactoyllactic acid and other polylactic acids, and water. It is usually in the form of the racemate, (RS)-lactic acid, but in some cases the (S)-(+)-isomer is predominant.
Being a carboxylic acid, lactate acid contributes hydrogen ions if a base is present to accept them. This occurs with both organic (for example, the amines) and inorganic bases. These reactions are named, “neutralizations”, and are coupled by the development of large amounts of heat. Neutralization between an acid and a base produces water plus a salt.
Speed up R&D and its documentation
Save R&D time and costs
Increase chances of approval and commercialization

