Compatible, Biodegradeable, Antibacterial, and Works in less than 5 Minutes
Chitosan, a natural biomaterial derived from the chitin shells of crustaceans and insects, has already been developed by scientists at Harvard’s Wyss Institute for Biologically Inspired Engineering into an environmentally-friendly and fully biodegradable substitute for plastic. It is only natural that the team, led by Wyss Institute Founding Director Donald Ingber, has also become interested in extending chitosan’s usefulness into the clinical realm.
“What’s good for the environment is also good for us,” said Javier Fernandez, who first developed a chitosan bioplastic called ‘Shrilk’ with Ingber in 2014.
Now Ingber and Fernandez have unveiled a new study in the journal Tissue Engineering that demonstrates biodegradable chitosan bioplastics can be used to bond bodily tissues to repair wounds or even to hold implanted medical devices in place in less than 5 minutes.
Natural biomaterials, such as chitosan and collagen, are commonly used for biomedical applications because they are biocompatible, mechanically rough yet flexible, and biodegradable. But it has been difficult to rapidly and firmly bond them to living tissues. In this study, the researchers demonstrate that the microbial transglutaminase (mTG), can be used to rapidly (<5 min) bond chitosan and collagen biomaterials to the surfaces of hepatic, cardiac, and dermal tissues, as well as to functionalized polydimethylsiloxane (PDMS) materials, a member of the silicone polymer family and used in medical products. The mTG-bonded chitosan patches effectively sealed intestinal perforations, and a newly developed two-component mTG-bonded chitosan spray effectively repaired ruptures in a breathing lung when tested ex vivo. The mechanical strength of mTG-catalyzed chitosan adhesive bonds were comparable to those generated by commonly used surgical glues. These results suggest that chitosan-mTG preparations may be broadly employed to bond various living tissue, which may open new possibilities for biomedical engineering, medical device integration, and tissue repair and regeneration.
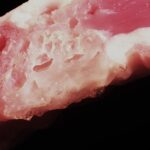
The chitosan foam material adheres so tightly to the muscle that its difficult to distinguish between chitosan foam and native tissue.
“As we started thinking about going in vivo, we faced the challenge of how to adhere chitosan to living tissues,” said Ingber, M.D., Ph.D., who is senior author on the new study and in addition to directing the Wyss Institute is Judah Folkman Professor of Vascular Biology at Harvard Medical School and the Vascular Biology Program at Boston Children’s Hospital as well as Professor of Bioengineering at Harvard’s John A. Paulson School of Engineering and Applied Sciences. “We explored using different formulations of transglutaminase to bond various forms of chitosan materials, including sheets, foams and sprays, to many different types of tissues.”
A sheet of chitosan may be applied with a transglutaminase powder to patch wounds, as the team demonstrated using an ex vivo porcine intestine with a large hole in it. A pressure test revealed that the chitosan patch was even stronger than the native intestinal tissue.
For the spray, a stream of liquid chitosan and liquid transglutaminase combine during application to quickly bond chitosan to tissue and close wounds. This approach was used to seal a porcine lung that had sustained a puncture wound while it was cyclically insufflated with air to mimic inspiration and expiration. The spray application could also be useful for covering large areas of vulnerable tissue, like might be found on someone whose skin had sustained serious burns.
To treat even larger and more traumatic wounds like those that might occur on the battlefield or during a motor vehicle accident, Ingber and Fernandez formulated a chitosan foam that could potentially be used to fill and seal larger wound cavities until a patient can be transported to a hospital for surgical intervention.
The team’s findings also suggest that these materials can also be engineered to bond inorganic surfaces, such as those used in biomedical implants and devices.
“Right now our approach is very general, but we could theoretically take this concept and adapt it into almost any form imaginable for a broad number of possible uses,” said Fernandez.
Looking ahead, the team hopes to develop an array of specific applications through collaboration with clinical partners.
Additional co-authors on the study include Wyss Institute researchers Suneil Seetharam, Christopher Ding, and Edward Doherty.
-
Wyss Institute for Biologically Inspired Engineering, Harvard University, Boston, Massachusetts.
-
Harvard School of Engineering and Applied Sciences, Harvard University, Cambridge, Massachusetts.
-
Vascular Biology Program, Department of Surgery, Children’s Hospital and Harvard Medical School, Boston, Massachusetts.
-
Vascular Biology Program, Department of Pathology, Children’s Hospital and Harvard Medical School, Boston, Massachusetts.



